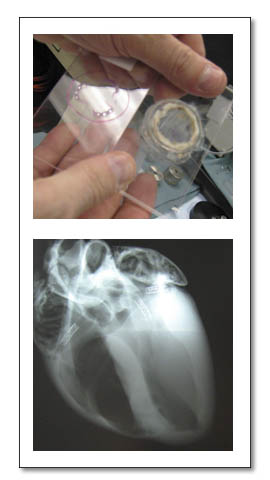
医療用具メーカーのコンサルタントAKIは、コンサルティングあるいはマーケティングノウハウを提供することで、医療用具メーカーの国際的な活動を支援します。 医療用具の海外動物試験海外承認申請GLP動物試験(米国)、CE mark取得、FDA申請など、日本のメーカーの側面支援を実施し、日本メーカーの海外進出を支援します。 医療用具の輸出入AKIは、欧州、米国、インド、中国などのメーカーの日本における市場調査、マーケティングの支援を実施します。 |
Business AffiliationsThe Import and Export of Medical Devices |
提携企業一覧 Business Affiliation & Company Information
|
Company information
AK International Co.,Ltd |
||||||